Alverno Laboratories Will Be First U.S. Network to Install Groundbreaking Digital Pathology Services from Philips
Alverno Laboratories, one of the largest laboratory networks in the United States, to launch groundbreaking digital pathology services from Royal Philips, a global leader in health technology. Once fully implemented by early 2020 Alverno Laboratories, located in northwest Indiana, will the single largest installed number of Philips scanners in the world.
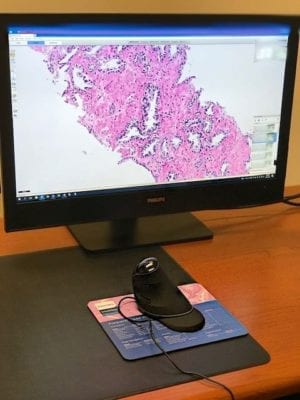
The laboratory network will use Philips IntelliSite Pathology Solution to assess and support the use of digital technology to interpret histology and diagnose pathology cases digitally, instead of using a microscope, with the aim of improving laboratory efficiency, quality and patient care and safety.
The scanners will allow for the “speedup” of diagnosis delivery for diseases like cancer as much as a day earlier, said Brian Wellborn, Alverno’s Manager of Anatomic Pathology.

Pictured: Sam Terese, CEO and Brian Wellborn, Pathology Manager
“This is transformational technology,” said Wellborn, who has been leading the project since April 2017. “Everything our pathologists do will be different now — in a good way — and we’ve had universally positive remarks about the changes. And this is going to improve turnaround time for patient diagnosis in a big way.”
The first sites to use the new technology for Phase 1 of the six-phase project will be: Franciscan Health Lafayette East in Lafayette, Indiana; Franciscan Health Dyer in Dyer, Indiana just east of the Illinois border; Franciscan Health Hammond in Hammond, Indiana; and Alverno’s core laboratory in Hammond, Indiana. Those sites will go live on June 15, Wellborn said.
The core laboratory site currently has four Philips Ultra Fast Scanners with four more to be installed on June 12, and four additional ones coming between January-February 2020, Wellborn said. The scanners replace traditional microscope technology to analyze slide tissue. Wellborn expects the scanners at Alverno Laboratories to process as many as 4,000 slides per day.

The hospital sites will receive highly specialized medical-grade monitors that will be used to quickly analyze information from tissue prepared at Alverno Laboratories’ central lab. The process eliminates the current courier service, which takes slides to the physical hospital sites from Alverno’s central laboratory. Hospital sites will also be able to share and collaborate on results with doctors at other hospital sites that employ the monitors, eliminating the need for courier service between hospitals.

“It eliminates problems with couriers that can be caused by weather, traffic and other issues,” Wellborn said. “This will make diagnoses quicker and more accurate as well.” The first phase of the project should be 100 percent digital by Aug. 15, with the full project finished by April 2020, Wellborn said.
Wellborn said Alverno is the first integrated laboratory network to receive the Philips technology in large part because of its CEO, Sam Terese. In April 2017, when reading a news release about the Philips technology being approved by the FDA, Terese printed it out and wrote a message to Wellborn that said “We’re Doing This.”
“Any time there’s a new technology, if it’s going to improve turnaround time and enhance the quality of care for our patients and pathologists, Sam will press forward,” Wellborn said.
The technology is currently being used by a handful of U.S. universities, but nothing the scope of Alverno Laboratories’.

Sam Terese, CEO of Alverno Laboratories
“Alverno is committed to ensuring our patients and clinical colleagues receive the fastest, most effective and best-informed diagnoses possible by employing the latest technology innovations,” Terese said. “Digital pathology enables enhanced cooperation and access to sub-specialists, helping us improve turnaround times and ultimately advancing our goal of saving lives.”
Wellborn, an Air Force veteran who lives in Bourbonnais, IL knows firsthand what it’s like to wait for a diagnosis. His father died of lung cancer, and Wellborn remembers waiting 4-5 days to see results of his father’s cancer screening.
“It’s a difficult situation for patients,” Wellborn said.
Wellborn said Alverno will be a key testing ground for Philips’ new technology.
“Philips in banking on us doing this correctly so they can bring this technology to other networks,” Wellborn said.
For more information on Alverno Laboratories, visit https://alvernolabs.com/.






